·Engaged in developing iron-based bioresorbable materials and devices since 2006
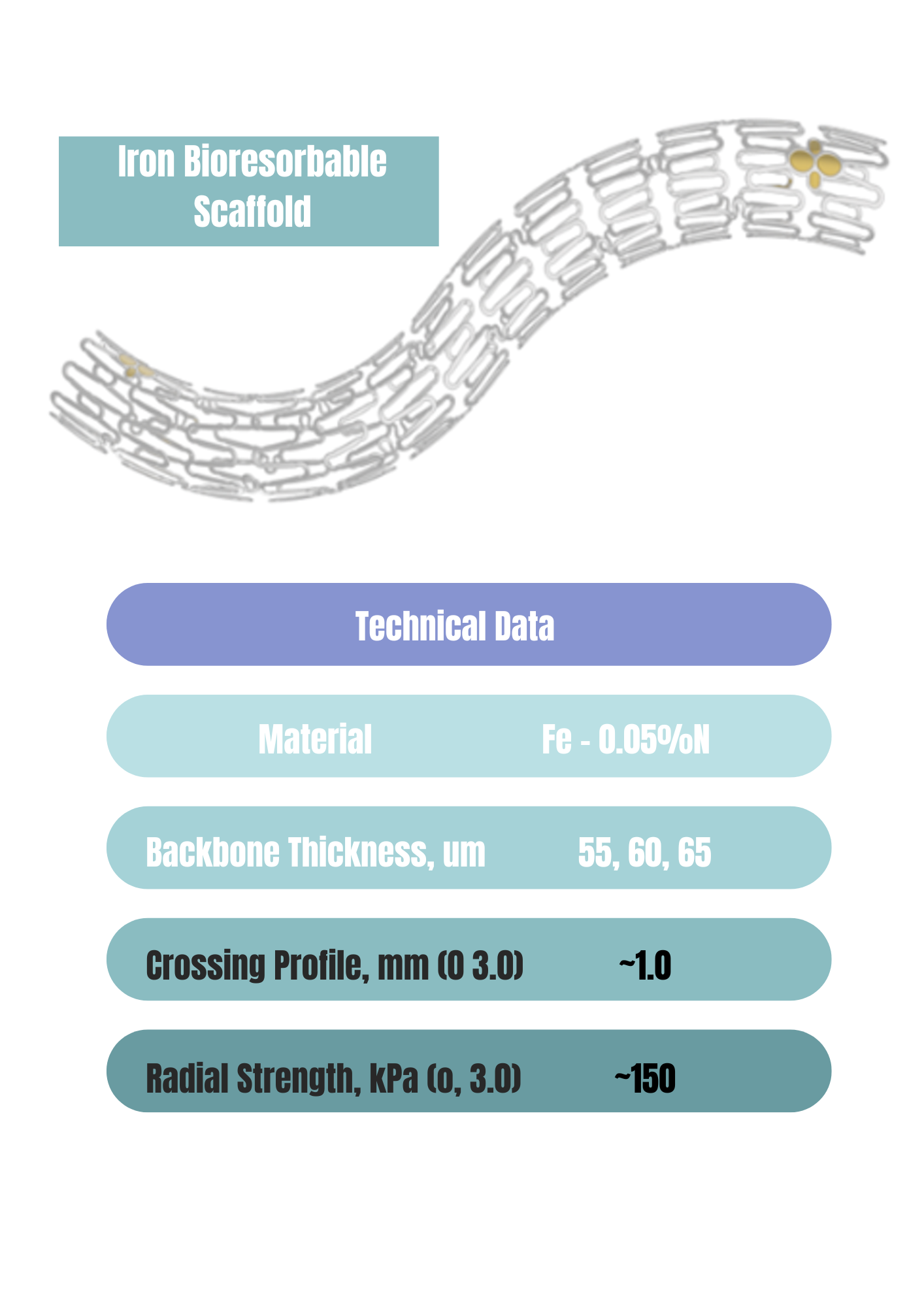
·Fe-0.05%N Backbone
·Strength to CoCr alloy
·Thinnest struts 55 μm
·Full sizing.
·Fast endothelialization
·Unique design and technical solution for controlled degradation and drug release,
·Biosafety
·Provide more possibilities for restenosis and interventional remediation of blood vessels.
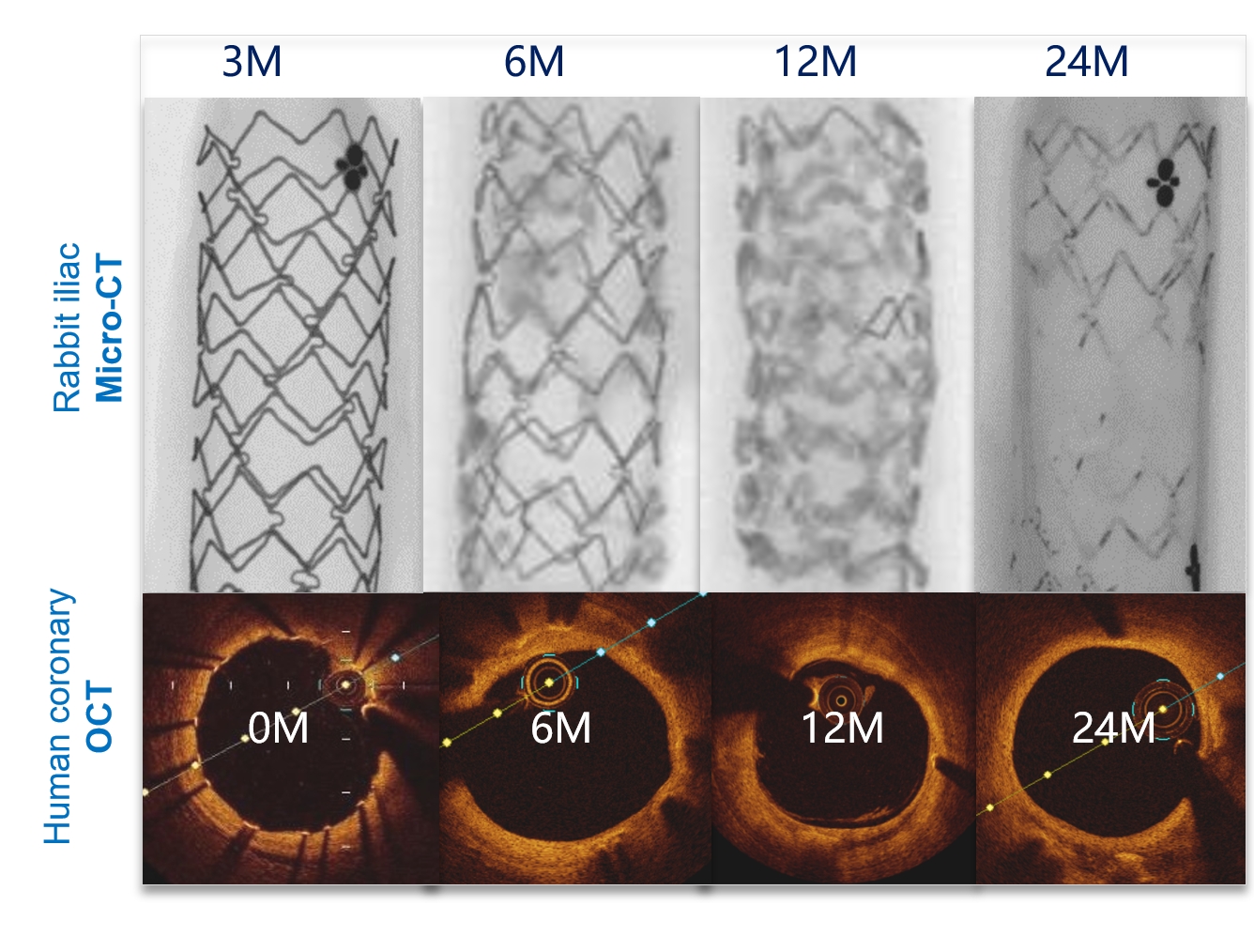
This treatment uses the nitrided process of the pure iron, adding 0.05% wt nitrogen to the iron to improve the mechanical properties of the scaffold to ensure that the scaffold provides effective support during the vascular recovery period. Compared with other BRSs, it has a thinner strut which facilitates rapid endothelialization, thereby reducing the rise of thrombosis.
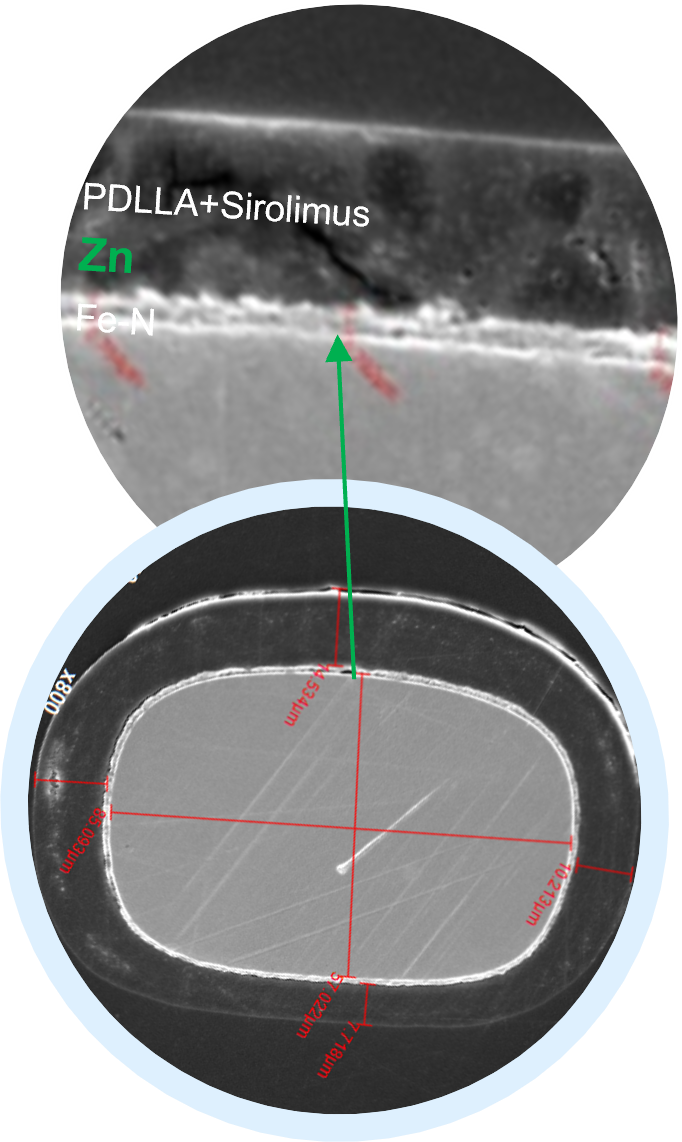
Considering that the implant must maintain its integrity for a certain period, we inserted a nanometer-thick layer of zinc between the polylactic acid and the iron. Zinc is used as a sacrificial anode to ensure that the iron backbone is completely non-degraded before it is absorbed to protect the iron backbone from degradation in the early stage, prolong the adequate support time, and facilitate blood vessel recovery.
We designed two gold radiopaque markers at both ends of the scaffold, which can help physicians complete precise positioning under DSA.
Testing has demonstrated that the IBS coronary scaffold is MR-compatible, which is the same as other permanent stents.
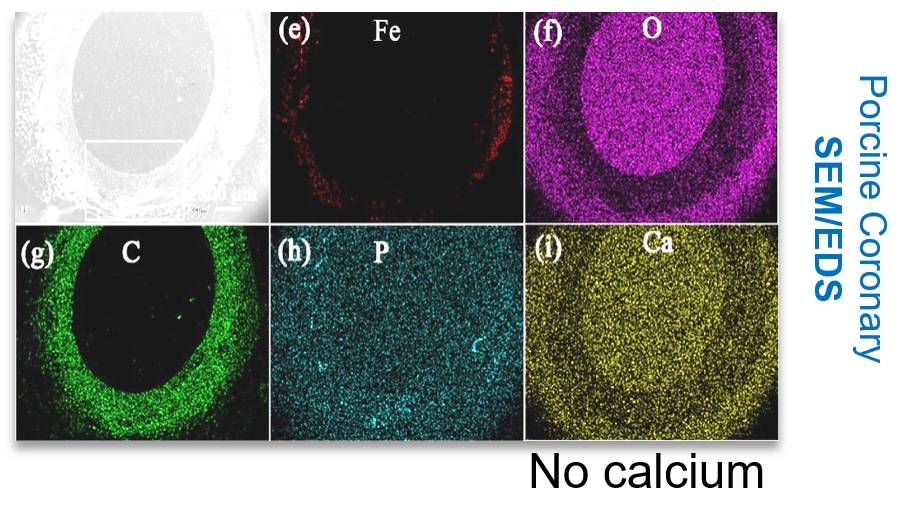
The corrosion products of the iron scaffold are biosafe and have good biocompatibility. SEM and EDS analysis show that the corrosion products of the iron scaffold do not contain calcium, which provides a safer and more effective solution for patients.